
Note after the electrons reach 32 they can't be accumulated in a shell. Atomic orbitals define the distribution of electrons in an elements electron configuration.

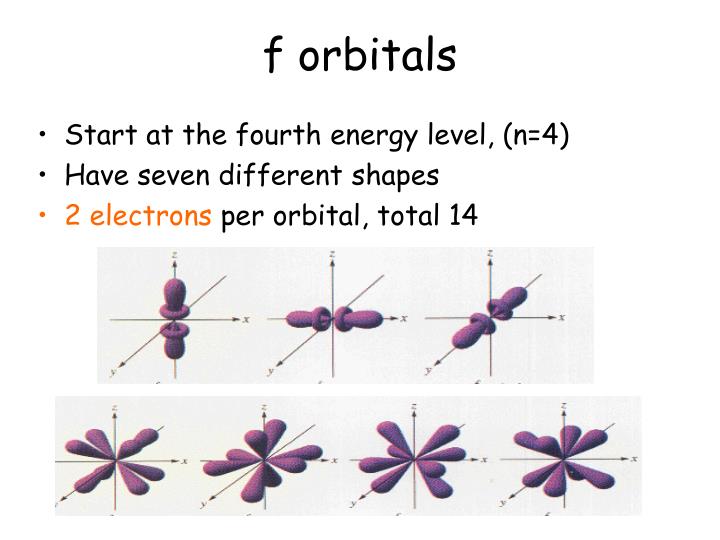
There is only one s orbital (m l 0), but there are three p orbitals (m l 1,0,1), ve d orbitals (m l 2,1,0,1,2), and seven f orbitals (m l 3,2,1,0,1,2,3). Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. Which type of Orbital has the highest energy within a shell? The s, p, d, f and g are called atomic orbitals. 12 Achievements Players must complete Challenge Levels 1 - 5 (Tutorial Levels) before unlocking the Sandbox. properties as a non-relativistic spatial orbital occupied by an electron. They were given the name halogen, from the Greek roots hal - (salt) and - gen (to produce), because they all produce sodium salts of similar properties, of which sodium chloridetable salt, or halite is best known. This loss in orbital energy should result in the electrons orbit getting continually smaller until it spirals into the nucleus, implying that atoms are. atomic size, electronegativity Sandbox The Sandbox is an exploratory learning space for extended practice and review of atoms. (ii) Interaction with the optimum halogen valence state 61. The s subshell is the lowest energy subshell and the f subshell is the highest energy subshell. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). The maximum number of electrons in d-orbital of an element with atomic number 46 is. x-ray spectroscopyanalysis of x rays emitted from a target hit by an electron beam (Instrumental Methods: X Rays, Atomic Numbers, and Orbital Structure. Since electrons all have the same charge, they stay as far away as possible because of repulsion.Īlso to know is, which Subshell contains the highest energy electrons?Įach subshell has a maximum number of electrons which it can hold: s - 2 electrons, p - 6 electrons, d - 10 electrons, and f - 14 electrons. Electrons that are in the highest energy level are called valence electrons.Īlso Know, which Orbital has the highest energy lowest energy? The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.

The larger the number of the energy level, the farther it is from the nucleus. Since the valence electrons are found in both s and p orbitals which have slightly different energies, the valence electrons of halogens are not in orbitals of the same energy level. Besides, which electron level has the highest energy?


 0 kommentar(er)
0 kommentar(er)
